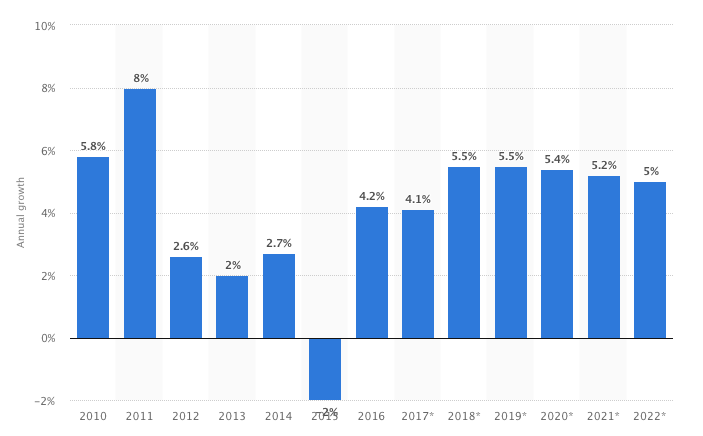
The global economic prospective looks promising for 2018. Medical technology sector showed signs of stability with the global growth at 4.1% in 2017 down from 4.2% within on year.
According to Statista the 2018 projections for the Metdtech industry will continue its growth trend with 25% increase establishing its pattern at 5.5% on average over ten year period.
But who are the key drivers inside Medtech industry ?
Minimum invasive surgery segment, cardiology in particular, is leading the trend along with medical imagery and point of care diagnostic solutions. Western world aging population market will see a growth on prevention analysis as healthcare is putting a gradually increasing strain on governments budget. Conversely the 300-million strong middle-class population, in India, with considerable disposable income, is rapidly growing. This well educated class of individuals is likely to demand better healthcare services and will play an important role in purchasing of healthcare services. Personal healthcare expenditure by households has grown from 4 percent to 7 percent during 1995 to 2005 and is expected to reach 13 percent in 2025. Moreover, as health insurance becomes an option of choice, it will also result in increased discipline and defined standards of care in hospitals.
Report Analysis
The uptake of innovative treatment technologies, including TAVR and 3D printing, is creating new cardiovascular treatment options and expanding the MedTech cardiovascular disease market – Deloitte
How the regulatory landscape will fashion the industry and what are the main challenges ahead ?
As the old saying goes, the only constant in life is change. For the medical device industry, over the past couple of years, this has certainly been the case. Changes will continue during 2018 and according to Jon Speer, one of industry experts. Here’s an inside of his predictions for 2018:
EU market will become more restrictive as new regulations will come into force.By contrast, the FDA model has come under some scrutiny in recent years. However, with the new administration, including a new head of FDA, there seems to be significant interest by FDA in simplifying the clearance process for life sciences technologies.
Healthcare Canada will back off on MDSAP deadline.Some of the biggest news in the medical device industry in 2017 was the medical device single audit program (MDSAP). Health Canada announced that companies selling medical devices into the Canadian market would be required to have ISO 13485:2016 (international standard for medical devices quality management systems) with MDSAP audit prior to 2019.
Medical device companies shift from compliance-focused to quality- focused. The most innovative and progressive medical device companies will escape the compliance spiral and shift to being quality focused. And those companies that make this shift to “true quality” will have huge, positive impact on quality of life.
Good news on shifting from compliance focused to quality focused with respect to FDA. FDA has been quietly promoting the “Case for Quality” program.
Coming to conclusion, medical device companies and medical technology sector as a whole will continue to be the focal point for investors worldwide.